A Monocentric Randomized, Double-Blind, Placebo-Controlled Trial on the Effectiveness of Bioarginina® in Oral Administration for the Treatment of Asthenia in Patients without Associated Comorbidities
Cuomo O. Simeone G. Minale M. Grieco F Amella C Alvisi V
DOI : https://doi.org/10.31546/IJFSNR.1007
Download Article : Peer-reviewed Article PDF
Abstract
In the last 6 decades a great debate about an idiopathic origin of moderate to severe asthenia was addressed by physicians. Asthenia is defined as a symptom of weakness related to different conditions. It can be associated to disabling symptoms such as sore throat, cervical and axillary lymphadenopathy, myalgia, arthralgia, migraine, concentration, memory and sleep disorders and malaise after physical efforts.
Arginine is a conditionally essential amino acid that is involved in protein synthesis, the detoxification of ammonia, and its conversion to glucose as well as being catabolized to produce energy; in addition to these physiological functions, arginine has shown to have ergogenic potential. Arginine-based supplement may be used on an acute basis for delaying the onset of neuromuscular fatigue (i.e., PWCFT) and improving the ventilatory threshold. The present research was conducted according to an experimental double-blind controlled design (vs. placebo), with the aim to evaluate the clinical efficacy and safety of the Bioarginina® (1.66g/20ml vials of L-arginine) in oral administration.
The changes in the parameters relevant to physical and/or mental fatigue, monitored for the evaluation of the efficacy of the experimental treatment, showed a clear difference in patients treated with Bioarginina® compared to those treated with placebo, with a statistically better trend in the Bioarginina® group than in the placebo group.
The general conditions, especially at the end of the observation period, improved significantly in the Bioarginina® group compared to the placebo group. Overall, asthenia symptoms had a marked improvement in patients in the Bioarginina® group, with a reduction of almost 25% of their intensity after 30 days of therapy and over 70% at the end of the treatment; by contrast, in the placebo group, the symptomatology underwent to an impairment, assessable in the order of 14% after one month and 25% at the end of the therapy. Overall the results of the present study strongly support the effectiveness of oral Bioarginina® for the treatment of symptoms associated to asthenia and highlight the possibility of a clinical application of Bioarginina® in the treatment of asthenia.
Keywords: Bioarginina®, Asthenia, Fatigue, L-arginine.
Introduction
In the last 6 decades a great debate about an idiopathic origin of moderate to severe asthenia was addressed by physicians. Asthenia is defined as a weakness symptom related to different conditions. It can be associated to disabling symptoms such as sore throat, cervical and axillary lymphadenopathy, myalgia, arthralgia, migraine, concentration, memory and sleep disorders and malaise after physical efforts [1]. This condition has been characterized, recognized, studied and classified in depth for the first time by K. Fukuda and colleagues in 1994 [2]. Chronic Fatigue Syndrome (CFS) is defined by severe fatigue of at least six months’ duration that interfere substantially with occupational, educational, social or personal activities. It is not alleviated by rest and cannot be explained by other medical and psychiatric conditions. For these reasons CFS imposes a significant burden on society and on people living with the illness [3]. A certain degree of asthenia accompanied by a sense of physical and/or psychological fatigue is often found in patients, both in those hospitalized, and in those who come to the observation of the general practitioner.
This symptomatology, far from being pathognomonic of neurological or neuropsychiatric diseases, can sometimes be an important indicator of an incoming organic disease, being itself a disabling and not always well tolerated condition for the patient. Most people experienced in their life a feeling of fatigue as an effect of intense or prolonged physical or intellectual effort. The consequences of this state are the difficulty in concentrating, the decreased work performance, the decreased interest in common activities and easy concern for even trivial details. A common mistake is the tendency to attribute the effects of an anxious neurosis or a latent depression to the overwork. These conditions, are generally characterized by distinctive elements (more frequent asthenia in the morning, acute need to rest, great difficulty in sleeping, frequent relationship between anxious state and an emotional event), allowing a differential diagnosis from physical-mental fatigue. Arginine is a conditionally essential amino acid that is involved in protein synthesis, the detoxification of ammonia, and its conversion to glucose as well as being catabolized to produce energy; in addition to these physiological functions, arginine has shown to have ergogenic potential [4]. Argininebased supplement may be used on an acute basis for delaying the onset of neuromuscular fatigue (i.e., PWCFT) and improving the ventilatory threshold [5].
The present research is a clinical study based on the evaluation of the effectiveness of Bioarginina [6] whose pharmacological and physiological properties which are known for many years [7-13], in the treatment of asthenia and fatigue. This study was conducted on patients enrolled by the Ferrara University Hospital “Sant’Anna†and then monitored or in outpatient care, according to an experimental double-blind controlled design (vs. placebo), with the aim to evaluate the clinical efficacy, general and local tolerability and safety of the Bioarginina (1,66g/20ml vials of L-arginine) after oral administration. This trial was supported by an institutional grant of Farmaceutici Damor SpA (Naples, Italy). The study was conducted in accordance with good clinical practice (G.C.P.), and with the principles of the Helsinki Declaration (1964) as amended in the revisions of Tokyo (1975), Venice (1983) and Hong- Kong (1989) and respecting the ethics and medical deontology. The clinical research, covered by insurance policy contracted by the Sponsor, was conducted after the approval of the Ethics Committee of the Hospital of Ferrara.
Patients and methods
Patients selection
200 patients were enrolled after a 15-day pharmacological wash-out. Patients were suffering asthenia associated with physical or mental fatigue, resulting from surgery or illness, intense physical or intellectual activity, or puerperium and lactation. All patients provided written informed consent before study participation. 94 patients were male and 106 female, aged between 18 and 69 (average 46.82 years). They underwent a balanced randomization to either the study drug or the placebo for ninety days. Patients who had severe renal impairment, hyperchloremic acidosis, patients suffering from serious infections, serious nervous diseases or neoplastic diseases, subjects with a history of alcohol or drug and those who underwent resuscitation or were unable to give their free and conscious adherence to the study for any reason were excluded. Furthermore all those patients who are suffering a condition or received, shortly before enrollment, a therapy that could interfere with the study treatment in the opinion of the investigator were also excluded.
Evaluation of patient general conditions
Patients general conditions were evaluated at all admissions and follow-up visits. These conditions have been summarized with a scale of values from 0 to 4 (0 = very bad, 1 = poor, 2 = mediocre, 3 = good, 4 = excellent). All patients were provided with a personal diary at the time of admission to indicate the intensity of some symptoms (weakness, physical and mental fatigue, lack of appetite, insomnia, daytime sleepiness, irritability, distractibility); the timing of the surveys was as follows: the day of the admission visit (t.-15), the day of treatment onset (t.0) and every fifteen days during the therapy (t.15, t.30, t .45, t.60, t.75 and t.90); for the intensity of each symptom a visuo-analog decimal scale was used, in which 0 indicates absence of symptoms and 10 stands for maximum intensity of symptoms.
In the last 6 decades a great debate about an idiopathic origin of moderate to severe asthenia was addressed by physicians. Asthenia is defined as a symptom of weakness related to different conditions. It can be associated to disabling symptoms such as sore throat, cervical and axillary lymphadenopathy, myalgia, arthralgia, migraine, concentration, memory and sleep disorders and malaise after physical efforts.
Arginine is a conditionally essential amino acid that is involved in protein synthesis, the detoxification of ammonia, and its conversion to glucose as well as being catabolized to produce energy; in addition to these physiological functions, arginine has shown to have ergogenic potential. Arginine-based supplement may be used on an acute basis for delaying the onset of neuromuscular fatigue (i.e., PWCFT) and improving the ventilatory threshold. The present research was conducted according to an experimental double-blind controlled design (vs. placebo), with the aim to evaluate the clinical efficacy and safety of the Bioarginina® (1.66g/20ml vials of L-arginine) in oral administration.
The changes in the parameters relevant to physical and/or mental fatigue, monitored for the evaluation of the efficacy of the experimental treatment, showed a clear difference in patients treated with Bioarginina® compared to those treated with placebo, with a statistically better trend in the Bioarginina® group than in the placebo group.
The general conditions, especially at the end of the observation period, improved significantly in the Bioarginina® group compared to the placebo group. Overall, asthenia symptoms had a marked improvement in patients in the Bioarginina® group, with a reduction of almost 25% of their intensity after 30 days of therapy and over 70% at the end of the treatment; by contrast, in the placebo group, the symptomatology underwent to an impairment, assessable in the order of 14% after one month and 25% at the end of the therapy. Overall the results of the present study strongly support the effectiveness of oral Bioarginina® for the treatment of symptoms associated to asthenia and highlight the possibility of a clinical application of Bioarginina® in the treatment of asthenia.
Keywords: Bioarginina®, Asthenia, Fatigue, L-arginine.
Introduction
In the last 6 decades a great debate about an idiopathic origin of moderate to severe asthenia was addressed by physicians. Asthenia is defined as a weakness symptom related to different conditions. It can be associated to disabling symptoms such as sore throat, cervical and axillary lymphadenopathy, myalgia, arthralgia, migraine, concentration, memory and sleep disorders and malaise after physical efforts [1]. This condition has been characterized, recognized, studied and classified in depth for the first time by K. Fukuda and colleagues in 1994 [2]. Chronic Fatigue Syndrome (CFS) is defined by severe fatigue of at least six months’ duration that interfere substantially with occupational, educational, social or personal activities. It is not alleviated by rest and cannot be explained by other medical and psychiatric conditions. For these reasons CFS imposes a significant burden on society and on people living with the illness [3]. A certain degree of asthenia accompanied by a sense of physical and/or psychological fatigue is often found in patients, both in those hospitalized, and in those who come to the observation of the general practitioner.
This symptomatology, far from being pathognomonic of neurological or neuropsychiatric diseases, can sometimes be an important indicator of an incoming organic disease, being itself a disabling and not always well tolerated condition for the patient. Most people experienced in their life a feeling of fatigue as an effect of intense or prolonged physical or intellectual effort. The consequences of this state are the difficulty in concentrating, the decreased work performance, the decreased interest in common activities and easy concern for even trivial details. A common mistake is the tendency to attribute the effects of an anxious neurosis or a latent depression to the overwork. These conditions, are generally characterized by distinctive elements (more frequent asthenia in the morning, acute need to rest, great difficulty in sleeping, frequent relationship between anxious state and an emotional event), allowing a differential diagnosis from physical-mental fatigue. Arginine is a conditionally essential amino acid that is involved in protein synthesis, the detoxification of ammonia, and its conversion to glucose as well as being catabolized to produce energy; in addition to these physiological functions, arginine has shown to have ergogenic potential [4]. Argininebased supplement may be used on an acute basis for delaying the onset of neuromuscular fatigue (i.e., PWCFT) and improving the ventilatory threshold [5].
The present research is a clinical study based on the evaluation of the effectiveness of Bioarginina [6] whose pharmacological and physiological properties which are known for many years [7-13], in the treatment of asthenia and fatigue. This study was conducted on patients enrolled by the Ferrara University Hospital “Sant’Anna†and then monitored or in outpatient care, according to an experimental double-blind controlled design (vs. placebo), with the aim to evaluate the clinical efficacy, general and local tolerability and safety of the Bioarginina (1,66g/20ml vials of L-arginine) after oral administration. This trial was supported by an institutional grant of Farmaceutici Damor SpA (Naples, Italy). The study was conducted in accordance with good clinical practice (G.C.P.), and with the principles of the Helsinki Declaration (1964) as amended in the revisions of Tokyo (1975), Venice (1983) and Hong- Kong (1989) and respecting the ethics and medical deontology. The clinical research, covered by insurance policy contracted by the Sponsor, was conducted after the approval of the Ethics Committee of the Hospital of Ferrara.
Patients and methods
Patients selection
200 patients were enrolled after a 15-day pharmacological wash-out. Patients were suffering asthenia associated with physical or mental fatigue, resulting from surgery or illness, intense physical or intellectual activity, or puerperium and lactation. All patients provided written informed consent before study participation. 94 patients were male and 106 female, aged between 18 and 69 (average 46.82 years). They underwent a balanced randomization to either the study drug or the placebo for ninety days. Patients who had severe renal impairment, hyperchloremic acidosis, patients suffering from serious infections, serious nervous diseases or neoplastic diseases, subjects with a history of alcohol or drug and those who underwent resuscitation or were unable to give their free and conscious adherence to the study for any reason were excluded. Furthermore all those patients who are suffering a condition or received, shortly before enrollment, a therapy that could interfere with the study treatment in the opinion of the investigator were also excluded.
Evaluation of patient general conditions
Patients general conditions were evaluated at all admissions and follow-up visits. These conditions have been summarized with a scale of values from 0 to 4 (0 = very bad, 1 = poor, 2 = mediocre, 3 = good, 4 = excellent). All patients were provided with a personal diary at the time of admission to indicate the intensity of some symptoms (weakness, physical and mental fatigue, lack of appetite, insomnia, daytime sleepiness, irritability, distractibility); the timing of the surveys was as follows: the day of the admission visit (t.-15), the day of treatment onset (t.0) and every fifteen days during the therapy (t.15, t.30, t .45, t.60, t.75 and t.90); for the intensity of each symptom a visuo-analog decimal scale was used, in which 0 indicates absence of symptoms and 10 stands for maximum intensity of symptoms.
Test Product Profile
Bioarginina contains 1.66g of L-arginine in 20ml vial for oral use and is freely marketed since May 1983. Placebo was totally similar to the vial and contained the same liquid of Bioarginina(R) without the L-Arginine. Bioarginina® or placebo was taken at the dosage of one vial per day. The study design is double blinded and randomized and contains 2 arms, experimental product vs placebo (1:1)
Bioarginina contains 1.66g of L-arginine in 20ml vial for oral use and is freely marketed since May 1983. Placebo was totally similar to the vial and contained the same liquid of Bioarginina(R) without the L-Arginine. Bioarginina® or placebo was taken at the dosage of one vial per day. The study design is double blinded and randomized and contains 2 arms, experimental product vs placebo (1:1)
Treatment Protocol The study included an admission visit at time 0, during which, each patient was monitored on a clinical level and the eventual symptomatology was recorded. Each patient received placebo for the first fifteen days (wash-out period), and then patients were subjected to the first control visit (baseline visit) to detect symptomatological and semiological parameters. Subsequently, patients were randomized to the oral Bioarginina or placebo arms. Symptomatology and semiological parameters were monitored after one and three months of treatment, as the final examination. In this context haematological and hematochemical tests were carried out and the symptomatological and neurological parameters were recorded and consequently all the data were analyzed for the conclusive judgments.
On the basis of the therapy used patients were randomized in two arms:
• BIOARGININA group - consisting of 50 males and 50 females, with an average age of 45.94 + 1.25 years (range from 19 to 68 years), an average height of 170.22 + 0.82 cm (range from 152 to 188 cm), and an average weight of 69.37 + 1.00 kg (range from 48.5 to 89 kg);
• PLACEBO group - consisting of 44 males and 56 females, with an average age of 47.70 + 1.31 years (range from 18 to 69 years), an average height of 168.47 + 0.88 cm (range from 153 to 185 cm), and an average weight of 68.51 + 1.05 kg (range from 51 to 90 kg). (see Table 1)
• BIOARGININA group - consisting of 50 males and 50 females, with an average age of 45.94 + 1.25 years (range from 19 to 68 years), an average height of 170.22 + 0.82 cm (range from 152 to 188 cm), and an average weight of 69.37 + 1.00 kg (range from 48.5 to 89 kg);
• PLACEBO group - consisting of 44 males and 56 females, with an average age of 47.70 + 1.31 years (range from 18 to 69 years), an average height of 168.47 + 0.88 cm (range from 153 to 185 cm), and an average weight of 68.51 + 1.05 kg (range from 51 to 90 kg). (see Table 1)
Toxicity Evaluation: At each check-up, any aggravation or onset of adverse events (signs or symptoms), both reported spontaneously by the patient and detected by the physician by objective and subjective examination, was detected. The time of appearance of each event, its duration and the intensity were specified, grading it from 0 = absent, 1 = mild, 2 = moderate, 3 = severe. The cause-effect correlation between the drug and the undesired event for any sign or symptom was recorded, using terms: unknown, non-existent, improbable, possible, probable, and certain. The investigator at his sole discretion decided whether to stop the treatment, reduce the dose, resort to specific symptomatic therapy or continue without variations.
Statistical analysis
The data were subjected to descriptive analysis such as mean ± SD and standard error, minimum and maximum value. Given the nature of the study, an analysis of variation over time was performed within group and between clinical and laboratory parameters, by analysis of variance, Student's test for paired data or for independent samples. The judgments and entities of the various signs or symptoms were instead subjected to non-test, the Wilcoxon test for group comparison, the Mann-Whitney U test for group comparison, or others if necessary. We considered the value of P <0.05 as a significance limit.
Results
Patients features: All patients admitted to the study completed the assigned treatment as required by the protocol. The patients admitted to the study, randomized into two experimental groups, were substantially homogeneous from a statistical point of view, and therefore comparable for diagnosis and all baseline parameters foreseen by the experimental protocol for the evaluation of the efficacy of the drug [Table 1].
 
Patients features: All patients admitted to the study completed the assigned treatment as required by the protocol. The patients admitted to the study, randomized into two experimental groups, were substantially homogeneous from a statistical point of view, and therefore comparable for diagnosis and all baseline parameters foreseen by the experimental protocol for the evaluation of the efficacy of the drug [Table 1].
 

Clinical efficacy
General conditions of the patients were significantly improved at the time of the last visit, in the intervention group, compared to the placebo group on day 90 of treatment, according to the data reported by patients themselves on their personal diary [Table 2A]. Data on body temperature and body weight of patients showed no change in both groups (data not shown). Even the trend of the systolic and diastolic blood pressure were comparable and constant over the 90 days of treatment in the two groups (data not shown). The trend in heart rate showed a slight increase in beats per minute in both experimental groups towards the end of the treatment period [Table 2B].



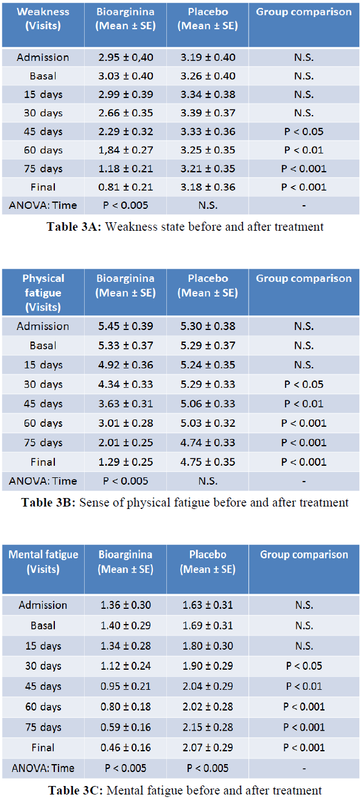
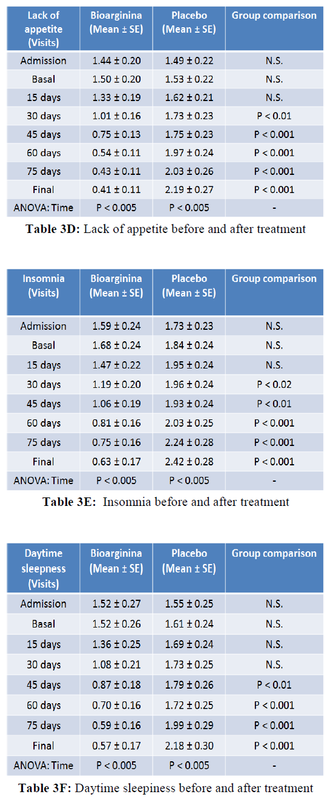
More important, the reduction of physical weakness, recorded by patients in their personal diary, was significantly improved starting from day 45 (half treatment) in the intervention group compared to the controls. More interestingly from the enrollment to the final visit a strong reduction in weakness of about 360% was observed in the Bioarginina treated group. By contrast, no reduction in weakness was observed in the control group [Table 3A]. The improvement in the sense of physical fatigue in the intervention group compared to the controls was observed as early as day 30 of treatment. In the Bioarginina group, from the enrollment to the final visit, a reduction in the sensation of physical fatigue of over 420% was detected. However, the slight reduction in the sense of physical fatigue observed in the control group was not statistically significant [Table 3B]. Furthermore, Bioarginina induced also an improvement of 290% in mental fatigue sensation from the enrolment to the final visit. The effectiveness of the treatment was also reinforced by the observation that, in contrast, the mental fatigue seems to worsen significantly in the control group from the enrollment to the final visit [Table 3C]. The same trend was found in the data about the lack of appetite and insomnia [Table 3D-E]. Daytime sleepness, irritability and distractibility improved significantly starting from day 45 visit and recorded improvements of 260%, 350% and 440% respectively within the Bioarginina group, from the enrollment to the final visit [Table 3F-G-H].
Toxicity: No toxicity and adverse events were reported in both groups. Furthermore, hematological and urine samples from both placebo and Bioarginina arms did not show any differences or issues.

Discussion
The changes in the parameters relevant to physical and/or mental fatigue, monitored for the evaluation of the efficacy of the experimental treatment, showed a clear difference in patients treated with Bioarginina compared to those treated with placebo, with a statistically better trend in the Bioarginina group than in the placebo group. The general conditions, especially at the end of the observation period, improved significantly in the Bioarginina group compared to the placebo group. Overall, asthenia symptoms had a marked improvement in patients in the Bioarginina group, with a reduction of almost 25% of their intensity after 30 days of therapy and over 70% at the end of the treatment; by contrast, as it was logical to expect, in the placebo group, the symptomatology underwent to an impairment, assessable in the order of 14% after one month and 25% at the end of therapy. With regard to the symptoms of physical fatigue, obtained by adding physical weakness and fatigue to each patient, a progressive improvement was observed in the Bioarginina group after an initial deterioration (due to the pre-enrollement pharmacological washout); after one month of Bioarginina therapy , the symptoms of physical fatigue were reduced by about 13%, while at the end of the therapy they regressed by over 65%. In the placebo group, on the other hand, the symptomatic profile became worse during the first two months (with an increase of more than 50%) and subsequently it showed a slight improvement; at the end of the placebo therapy the symptoms of physical fatigue were worsened by more than 45% compared to the initial intensity.
The changes in the parameters relevant to physical and/or mental fatigue, monitored for the evaluation of the efficacy of the experimental treatment, showed a clear difference in patients treated with Bioarginina compared to those treated with placebo, with a statistically better trend in the Bioarginina group than in the placebo group. The general conditions, especially at the end of the observation period, improved significantly in the Bioarginina group compared to the placebo group. Overall, asthenia symptoms had a marked improvement in patients in the Bioarginina group, with a reduction of almost 25% of their intensity after 30 days of therapy and over 70% at the end of the treatment; by contrast, as it was logical to expect, in the placebo group, the symptomatology underwent to an impairment, assessable in the order of 14% after one month and 25% at the end of therapy. With regard to the symptoms of physical fatigue, obtained by adding physical weakness and fatigue to each patient, a progressive improvement was observed in the Bioarginina group after an initial deterioration (due to the pre-enrollement pharmacological washout); after one month of Bioarginina therapy , the symptoms of physical fatigue were reduced by about 13%, while at the end of the therapy they regressed by over 65%. In the placebo group, on the other hand, the symptomatic profile became worse during the first two months (with an increase of more than 50%) and subsequently it showed a slight improvement; at the end of the placebo therapy the symptoms of physical fatigue were worsened by more than 45% compared to the initial intensity.
In parallel, also the psycho-physical symptomatology showed a different evolution in the two arms: the strongly positive trend in the Bioarginina group mirrored with the negative trend in the placebo group. One month after Bioarginina treatment an average reduction in symptom’s intensity of about 23% was observed. This symptom improvement continues until the end of the treatment the drop when it reached 58% reduction. In the control group the increase was instead estimated in the order of 18% after one month and 43% at the end of therapy. The overall efficacy judgment was therefore markedly positive in the Bioarginina group, with more than 80% judgments of excellent, good or satisfactory efficacy, while in the placebo group the positive assessments did not reach 15%. Furthermore, Bioraginina resulted to be safe since no adverse reaction were reported. Overall the results of the present study strongly support the effectiveness of oral Bioarginina for the treatment of symptoms associated to asthenia and highlight the possibility of a clinical application of Bioarginina in the treatment of asthenia.
Conflict of Interest: The authors have no conflicts of interest to declare.
References
1. Conti F, Priori R, De Petrillo G, et al. Prevalence of chronic fatigue syndrome in Italian patients with persistent fatigue. Ann Ital Med Int. 1994; 9:219-222.
2. Fukuda K, Straus SE, Hickie I, et al. The chronic fatigue syndrome: a comprehensive approach to its definition and study. International Chronic Fatigue Syndrome Study Group. Ann Intern Med. 1994 ;12:953-959.
3. Solomon L, Reeves WC. Factors influencing the diagnosis of chronic fatigue syndrome. Arch Intern Med. 2004 ;164:2241-2245.
4. Campbell BI, La Bounty PM, Roberts M. The ergogenic potential of arginine. J Int Soc Sports Nutr. 2004 ;1:35-38.
5. Zak RB1, Camic CL, Hill EC, et al. Acute effects of an arginine-based supplement on neuromuscular, ventilatory, and metabolic fatigue thresholds during cycle ergometry. Appl Physiol Nutr Metab. 2015 ; 40:379-385.
6. James E.F. Reynolds . Martindale The Extra Pharmacopoeia. 29th Edition. The Pharmaceutical Press. Arginine . p. 1254, 1989.
7. Barbul, A. Arginine: biochemistry, physiology, and therapeutic implications. J Parent Ent Nutr. 1986. 10:227-238.
8. R. L. Prior, A. J. Clifford, D. E. Hogue, W. J. Visek. Enzymes and metabolites of intermediary metabolism in urea-fed sheep. J Nutr. 1970; 100:438-444.
9. Ellington WR. Phosphocreatine represents a thermodynamic and functional improvement over other muscle phosphagens. J Exp Biol. 1989 ;143:177-1794.
10. E. M Boyd, Carl E Boyd. Toxicity Of Pure Foods. 1st Edition. CRC Press. p. 139. 1973.
11. Piraino G. Rassegna internazionale di clinica e terapia. Napoli. 1985; 5.
12. Piraino G. Stampa Medica Europea. 1986; 5.
13. Albertini P. Quaderni di Medicina e Chirurgia (Excerpta Medica). 1987; 2.
1. Conti F, Priori R, De Petrillo G, et al. Prevalence of chronic fatigue syndrome in Italian patients with persistent fatigue. Ann Ital Med Int. 1994; 9:219-222.
2. Fukuda K, Straus SE, Hickie I, et al. The chronic fatigue syndrome: a comprehensive approach to its definition and study. International Chronic Fatigue Syndrome Study Group. Ann Intern Med. 1994 ;12:953-959.
3. Solomon L, Reeves WC. Factors influencing the diagnosis of chronic fatigue syndrome. Arch Intern Med. 2004 ;164:2241-2245.
4. Campbell BI, La Bounty PM, Roberts M. The ergogenic potential of arginine. J Int Soc Sports Nutr. 2004 ;1:35-38.
5. Zak RB1, Camic CL, Hill EC, et al. Acute effects of an arginine-based supplement on neuromuscular, ventilatory, and metabolic fatigue thresholds during cycle ergometry. Appl Physiol Nutr Metab. 2015 ; 40:379-385.
6. James E.F. Reynolds . Martindale The Extra Pharmacopoeia. 29th Edition. The Pharmaceutical Press. Arginine . p. 1254, 1989.
7. Barbul, A. Arginine: biochemistry, physiology, and therapeutic implications. J Parent Ent Nutr. 1986. 10:227-238.
8. R. L. Prior, A. J. Clifford, D. E. Hogue, W. J. Visek. Enzymes and metabolites of intermediary metabolism in urea-fed sheep. J Nutr. 1970; 100:438-444.
9. Ellington WR. Phosphocreatine represents a thermodynamic and functional improvement over other muscle phosphagens. J Exp Biol. 1989 ;143:177-1794.
10. E. M Boyd, Carl E Boyd. Toxicity Of Pure Foods. 1st Edition. CRC Press. p. 139. 1973.
11. Piraino G. Rassegna internazionale di clinica e terapia. Napoli. 1985; 5.
12. Piraino G. Stampa Medica Europea. 1986; 5.
13. Albertini P. Quaderni di Medicina e Chirurgia (Excerpta Medica). 1987; 2.
© 2018 medicalpressopenaccess.com. All rights reserved.



